 Viruses, with only a few genes, are able to commandeer the complex mechanisms of human cells. Previous posts have described remarkable behavior of herpes, HIV and Ebola. These tiny pieces of genetic material are able to make proteins that evade the attacks from immune cells and trick membranes to allow entry. Viruses can hide in plain site and edit pieces of RNA as weapons.
Viruses, with only a few genes, are able to commandeer the complex mechanisms of human cells. Previous posts have described remarkable behavior of herpes, HIV and Ebola. These tiny pieces of genetic material are able to make proteins that evade the attacks from immune cells and trick membranes to allow entry. Viruses can hide in plain site and edit pieces of RNA as weapons.
Now, it has been demonstrated that viruses use special proteins to alter the critical scaffolding molecules that produce all cellular structures, mobility and function. These virus tricks manipulating the cytoskeleton allow easy entry into the cell, and later the nucleus, as well as a takeover of the genetic machinery. The scaffolding molecules of the cells are critical for every aspect of cellular function, including neuroplasticity. Previous posts have demonstrated the vital work of microtubules in axon transport, and actin with myosin motors in neuroplasticity. This post describes how viruses are able to manipulate actin structures at every phase of the virus life cycle. These subversion techniques using actin vary tremendously with different viruses.
Basic Actin
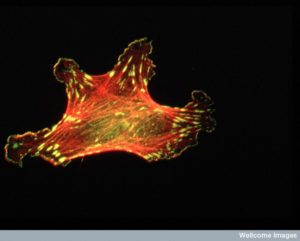
 There are three major fibers in the scaffolding skeletons and highways of all cells. The largest of the fibers are microtubules that form hollow tubules for structure and transport (See post Enormous Complexity of Transport Along the Axon.) The medium sized filaments are made of many different proteins—keratins in skin cells, lamins for a mesh inside the nuclear membrane, neurofilaments in neuronal axons, and vimentins providing strength in muscle. Actin, the smallest fiber, provides for the shape and mobility of the cell and all organelles. All of these are critical for neuroplasticity and there are many different motors that work with these filaments.
There are three major fibers in the scaffolding skeletons and highways of all cells. The largest of the fibers are microtubules that form hollow tubules for structure and transport (See post Enormous Complexity of Transport Along the Axon.) The medium sized filaments are made of many different proteins—keratins in skin cells, lamins for a mesh inside the nuclear membrane, neurofilaments in neuronal axons, and vimentins providing strength in muscle. Actin, the smallest fiber, provides for the shape and mobility of the cell and all organelles. All of these are critical for neuroplasticity and there are many different motors that work with these filaments.
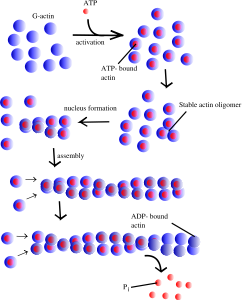
Actin is a globular protein that combines to form two types of filaments.The globule is made of two lobes with an energy ATP/ADP in the middle. This energy particle helps the globule combine with others to form many different filament structures. One type of filament is called the microfilament used in neurons and many other cells. The other are “thin” filaments used for muscle contraction working with myosin motors. (See post on Another Type of Neuroplasticity with Myosin Motors).
One actin molecule is called globular actin or G-actin. Actin globules combine to form linear filaments called filament actin or F-actin. There are three types of actin filaments, one in muscle and the other two for all other functions. One end the actin filament is positive.
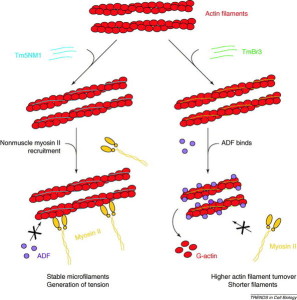
Many complex proteins work with actin to produce its many functions. One large family of proteins, called tropomyosins regulates the function of actin filaments in both muscle and non-muscle cells. These proteins consist of rod-shaped coiled molecules.
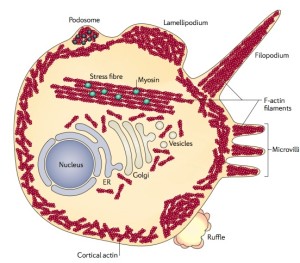
 Actin filaments form a mesh just under the cellular membrane called the cortical network. It links receptor proteins that lie across the membrane to the molecules inside the cell. These receptors can connect with microbes outside the cell and form a communication between the outside the cell and the cytoskeleton structure inside the cell. In this way actin with myosin motors can drag the virus that is still outside the cell to a better spot for entry.
Actin filaments form a mesh just under the cellular membrane called the cortical network. It links receptor proteins that lie across the membrane to the molecules inside the cell. These receptors can connect with microbes outside the cell and form a communication between the outside the cell and the cytoskeleton structure inside the cell. In this way actin with myosin motors can drag the virus that is still outside the cell to a better spot for entry.
The cortical mesh consists of actin and many molecules that attach to the membrane. There are no organelles in this mesh. Different attachments produce either flat or finger like shapes in the membrane. There are a large number of proteins and an equally large number of matrixes for very different cellular shapes.
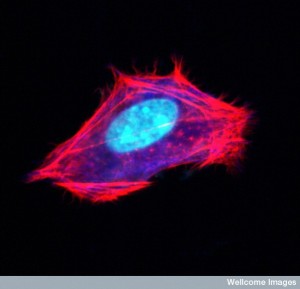
Actin, also, forms many different structures that are critical to cellular shape changes and cellular and organelle movement of all kinds. They are critical for junctions between cells, such as occur in skin, intestine and many other cells.
 An important actin filament is the large stress fiber. These are long strong fibers with cross links that provide strength to the cell’s shape and for cellular movement and migration, cell adhesions and cell shape changes. Stress fibers are like the major beams in buildings extending across the entire structure. They can contract using myosin motors for cell movement and function (One example of shrinking cells was described in neurons, when their size decreases by half during sleep in order to increase the cleaning flow to eliminate misfolded proteins in the extracellular space and protect from neurodegenerative illness – see post Five Secrets of Brain Health).
An important actin filament is the large stress fiber. These are long strong fibers with cross links that provide strength to the cell’s shape and for cellular movement and migration, cell adhesions and cell shape changes. Stress fibers are like the major beams in buildings extending across the entire structure. They can contract using myosin motors for cell movement and function (One example of shrinking cells was described in neurons, when their size decreases by half during sleep in order to increase the cleaning flow to eliminate misfolded proteins in the extracellular space and protect from neurodegenerative illness – see post Five Secrets of Brain Health).
Actin forms many out pouches from the membrane. In neurons, these include dendrites, dendrite spines and axon boutons. They, also, form many kinds of filopodia, podosomes, lamillipodia, microvilli and ruffles.
Actin Cytonemes
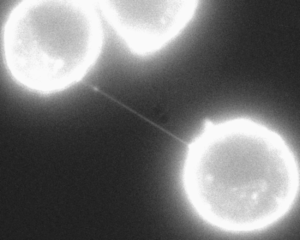
An important dramatic new finding is nanotubes made out of actin that project long distances between most cells. These cytonemes are a newly discovered vital communication conduit for cellular signaling. It is a filopodium of small size made of parallel actin filaments surrounded by membrane. They can be as long as ten cell lengths. There are, also, larger filopodium that are constructed from both actin and microtubules, and can transmit larger cargo such as vesicles and organelles.
Important new research shows that these nanotubes between most cells are the way that viruses can spread rapidly between cells. It was always thought that viruses mainly spread by coming out of one cell and then going into another. But, this does not explain the speed with which viruses spread. It now appears that the nanotubes are the major mechanism.
Actin is Vital for All Cellular Movement and Change
 All aspects of cellular movement, including the ability of cells to gobble up bacteria and debris (phagocytosis) are completely dependent upon the changing actin skeleton. The movement of white blood cells throughout the body, which is fueled by changing actin structures, has been described in a previous post.
All aspects of cellular movement, including the ability of cells to gobble up bacteria and debris (phagocytosis) are completely dependent upon the changing actin skeleton. The movement of white blood cells throughout the body, which is fueled by changing actin structures, has been described in a previous post.
Special enzyme complexes add more molecules to the actin filament, making the actin longer and longer. One complex (ARP2/3) has a special relationship with viruses. Particular proteins stimulate the complex. Two other proteins help the strand grow longer and strengthen it—profilin and cortactin. The proteins that make it smaller are cofilin and gelsolin (cuts actin filaments). When actin filaments become larger structures they use other proteins called fascins. When the structures has branches and cross links, the protein is actin-nucleating protein. With the motor myosin actin fibers contract, such as in muscle cells. Other actin structures hold the shape of the cell together just under the membrane and near organelles such as the endoplasmic reticulum.
When cells move, they use many different actin structures, such as filopodia and podosomes. They can make other structures for the leading edge of the cell, such as sheets of membrane called lamelipodia. Another moving structure looks like a flower and is called membrane ruffles. Large finger like protrusions are called pseudopodia.
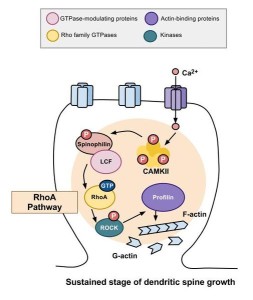
Actin structures are constantly changing as the cell moves and functions—building, breaking down and rebuilding in new ways. Signals from receptors in the membrane direct the changes of actin structures through a series of more than twenty different enzymes called RHO GTPases. These regulate creation of membranes and movement and polarity of cells.
Viruses are particularly focused on manipulating the RHO enzymes. Proteins from viruses alter the actin structures and their functions. It is quite remarkable that viruses can subvert any structure in their way, while they reproduce themselves.
Virus Effects on Actin
 The first observed effects of adenoviruses on cells were changes in cell shape, such as becoming more rounded and having more pseudopodia; although the cells had new pseudopodia, they were unable to move. Affected cells divided more frequently and piled up on top of each other. Cells did not have the usual junctures between them. Some of these changes can occur naturally in a dividing cell, such as the more rounded shape.
The first observed effects of adenoviruses on cells were changes in cell shape, such as becoming more rounded and having more pseudopodia; although the cells had new pseudopodia, they were unable to move. Affected cells divided more frequently and piled up on top of each other. Cells did not have the usual junctures between them. Some of these changes can occur naturally in a dividing cell, such as the more rounded shape.
The cortical mesh, just under the membrane, becomes rigid when affected by adenovirus. These changes are, also, part of a cell becoming cancerous, including contacting other cells and multiplying. Adenoviruses produced proteins that altered cells—first producing ruffles, then a rounded and retracted cell shape and then many microvilli, spikes and blebs. Virus proteins destroyed large stress fibers that were determining the cell’s shape. Virus protein was observed alongside other cellular proteins that alter the actin structures—vinculin (adhesion), talin (actin membrane interaction), cortactin and actinin (bundle filaments).
 The virus proteins act through phosphorylation of the important cellular enzymes vinculin and cofilin. Normally, cellular enzymes phosphorylate these enzymes. The structures that immune cells use to travel throughout the body are based on these many different actin structures such as podosomes and these enzymes. Podosomes are very complex actin structures that are used with myosin motors and surrounded by many other special proteins and metalloproteinases (metalloproteins produce electricity – see post Electric DNA). These respond to cytokines and other factors to remodel the actin skeleton and the extracellular matrix to allow the immune cells to move about and enter tissues. The podosomes of cancer are different and are not as mobile. These are called invadopodia and produce invasive cancer behavior.
The virus proteins act through phosphorylation of the important cellular enzymes vinculin and cofilin. Normally, cellular enzymes phosphorylate these enzymes. The structures that immune cells use to travel throughout the body are based on these many different actin structures such as podosomes and these enzymes. Podosomes are very complex actin structures that are used with myosin motors and surrounded by many other special proteins and metalloproteinases (metalloproteins produce electricity – see post Electric DNA). These respond to cytokines and other factors to remodel the actin skeleton and the extracellular matrix to allow the immune cells to move about and enter tissues. The podosomes of cancer are different and are not as mobile. These are called invadopodia and produce invasive cancer behavior.
Virus protein alters the entire use of actin so that the critical stress structures are not formed at all. Several important enzymes are not even manufactured due to the virus proteins. Somehow, there are, also, less collagen and fibronectin in ECM. In several viruses, one protein is responsible for loss of actin and alteration of critical structures.
 Virus proteins can stimulate the entire cell reproduction cycle and stop the critical structural stress factors. These changes create protrusions that are used for movement, so cells that are ordinarily attached and stationary become mobile and reproductive.
Virus proteins can stimulate the entire cell reproduction cycle and stop the critical structural stress factors. These changes create protrusions that are used for movement, so cells that are ordinarily attached and stationary become mobile and reproductive.
Herpes viruses are long lived in a large number of people, existing quietly in neurons and B-lymphocytes. Lymphocytes become abnormal in that they transform their shape, multiply frequently and can become cancerous. They have much more actin and tubulin and in unusual places, such as the surface of the cells. Actin is almost always inside the cell and this transport to the extracellular region is unique and its function is unknown. Special proteins, fascins, help actin form structures, by producing 200 times the usual amount of actin, which may increase these unusual protrusions. Another protein from herpes is a genetic transcription factor triggering these changes.
Hepatitis B virus alters liver cells shape and function by a special protein (protein X) by rearranging actin structures into lamellipodia and ruffles. This protein increases the process that copies the virus. These cells become abnormally mobile and often become cancerous.
 HIV virus produces two proteins that cause cancer, again by altering the cell cycle through the actin structures. These affect RHO pathways and increase p27, related to mobility and metastatic behavior. By decreasing RHO signals causing the cell to move. It is the actin motors that make the cell move.
HIV virus produces two proteins that cause cancer, again by altering the cell cycle through the actin structures. These affect RHO pathways and increase p27, related to mobility and metastatic behavior. By decreasing RHO signals causing the cell to move. It is the actin motors that make the cell move.
Several viruses produce RNA that are unusual types of oncogenes. The virus stimulates the cell to reproduce endlessly and the virus then lives in these cells.
There are a wide variety of different mechanisms that different viruses use to manipulate actin:
- Breaking up filaments,
- Stopping polymerization
- Blocking polymerization and increase breakup
- Increasing nucleation of actin
- Stoping uptake of monosaccharide
- Stabilizing actin structures and assemblies
- Competing with new actin addition
- Inhibiting myosin motors
- Allowing the crossing of membrane
- Inhibiting many cellular pathways
- Aiding virus replication
How the Virus Enters the Cell
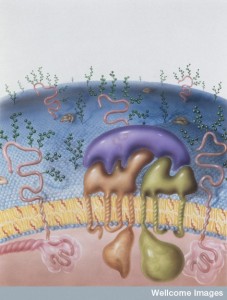 To enter the cell a virus must travel through the membrane and then navigate the cortical actin matrix. Remarkably, from outside the cell, the virus is able to stimulate the proteins to remodel the matrix.
To enter the cell a virus must travel through the membrane and then navigate the cortical actin matrix. Remarkably, from outside the cell, the virus is able to stimulate the proteins to remodel the matrix.
Viruses make a contact at the membrane surface with a glycoprotein that sticks through the membrane and is attached to the underlying actin structure. At the base of the filopodia, myosin motors pull the large actin filaments. More actin is being created at the tip, pushing the same filaments downward toward the myosin. This pulls the virus downward to where it can enter the cell. It can, also, pull them sideways to the entry receptor. This occurs by stimulating pathways in the actin below the surface through this contact.
For entry by clathrin endocytosis, the actin makes the region of the fusion pore bigger after being triggered by virus fusion proteins. While outside the cell and attempting to enter it, proteins from the cell direct the virus particle to parts of the membrane that are normally hidden, but allow entry through fusion (membrane bound viruses) and endocytosis (for naked genetic particles). This site is highly connected to the actin structures just below the membrane. The myosin motors in the actin help the virus find the exact site.
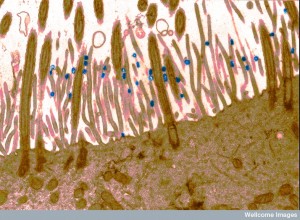 The actin scaffold actually grabs and moves the virus to the regions where entry can occur. This occurs in many viruses, including HIV and HPV. If the actin is disrupted below, then the virus doesn’t enter. The exact mechanism is not known, but a particular molecule related to pathways below the membrane is the lipid phophatidylserine, which triggers endocytosis through several pathways. Multiple changes in the actin skeleton occur in this complex process.
The actin scaffold actually grabs and moves the virus to the regions where entry can occur. This occurs in many viruses, including HIV and HPV. If the actin is disrupted below, then the virus doesn’t enter. The exact mechanism is not known, but a particular molecule related to pathways below the membrane is the lipid phophatidylserine, which triggers endocytosis through several pathways. Multiple changes in the actin skeleton occur in this complex process.
Several viruses without membrane envelopes use a particular receptor on the cell in the tight junctions between cells such as the skin. Normally, this receptor is hidden from view of the virus. This piece of DNA or RNA attach to the glycophosphatidylinositol instead, which is more easily available. This triggers a pathway below that alters the actin scaffold. Then the virus move along the surface directed to the junction where the receptor triggers entry though complex endocytosis.
HIV has an additional special mechanism. While looking for the entry point, they are able to stimulate more receptors (CD4 and CXCR4) to create clusters on the surface that enhance entry. These stimulate RHO and remodel the entire actin skeleton underneath through filamin and cofilin. Specific signals allow rapid growths of new actin structures.
Actin Affecting Endocytosis
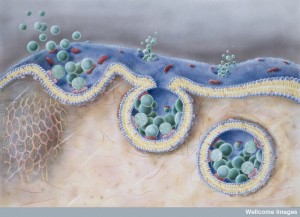 Drugs that upset the actin skeleton can stop virus entry. Ebola entry is through RHO stimulating an unusual macropinocytosis (see post on the Very Intelligent Ebola Virus). The complex mechanism involves many changes in actin skeleton. After entry, the poliovirus is quickly moved to the region of the cell where multiplication occurs through actin.
Drugs that upset the actin skeleton can stop virus entry. Ebola entry is through RHO stimulating an unusual macropinocytosis (see post on the Very Intelligent Ebola Virus). The complex mechanism involves many changes in actin skeleton. After entry, the poliovirus is quickly moved to the region of the cell where multiplication occurs through actin.
Viruses surrounded by a membrane envelope don’t use the endocytic system. These merge with the membrane using a glycoprotein and a fusion protein. Again, actin alterations make this happen.
Another mechanism for entry is making extensions of the cell surface, such as the protrusions mentioned. Phagocytosis can be triggered, bringing the virus into the cell by making extensions and creating clusters of receptors. The extensions need very long actin structures such as stress fibers. Some break up the stress fibers allowing the creation of rapid protrusions such as filopodia. The virus can enter in the filopodia; in fact, it attracts any virus particles that are nearby to enter.
Copying and Assembling Virus
To copy a virus requires a very complex coordinated process with multiple large proteins that transcribe the genetic material into usable messenger RNA and then translate this into virus proteins. Another process copies the virus DNA or RNA. To coordinate this activity, elaborate actin cytoskeletons are produced and altered during different stages of the process.
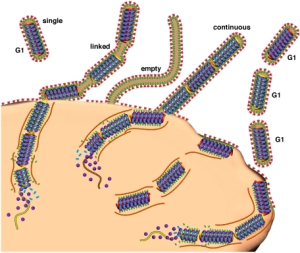 After the virus enters the cell, actin filaments and their myosin motors help the virus travel to the centers of reproduction at the nucleus. Actin, also, helps the virus get into the nucleus through the pore. At the cell surface, structures hold the pieces in place while the virus is wrapped in membrane.
After the virus enters the cell, actin filaments and their myosin motors help the virus travel to the centers of reproduction at the nucleus. Actin, also, helps the virus get into the nucleus through the pore. At the cell surface, structures hold the pieces in place while the virus is wrapped in membrane.
Assembly near the membrane uses a complex of proteins that forms buds in the membrane. These are connected with lipid rafts in the membrane and the process is triggered by actin alterations.
Some DNA viruses (herpes) make their capsids (covers) in the cell’s nucleus. They move to the membrane of the nucleus by actions of actin structures. The infections makes many more actin filaments and myosin motor V, which allows the capsid to move out of the nucleus. After the virus appears from the nucleus it, also, moves to the membrane and out of the cell by actin mechanisms with myosin motors interacting with the actin cortical network.
Getting out of the Cell
Getting out of the cell reverses the problems of getting in. Viruses must move rapidly from the nucleus region to the outer membrane and negotiate the actin cortical mesh.
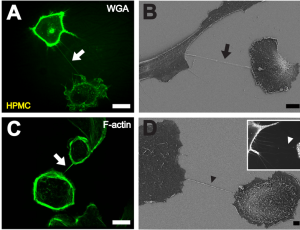 A previous post described nanotubes that exist between most cells. These cytonemes have been recently found to be in all cells, but because they are so small they have not been observed until recently. These are called cytonemes and are used for transport of genetic material proteins and viruses between cells.
A previous post described nanotubes that exist between most cells. These cytonemes have been recently found to be in all cells, but because they are so small they have not been observed until recently. These are called cytonemes and are used for transport of genetic material proteins and viruses between cells.
The rapid spread of viruses from cell to cell occurs in these nanotube cytonemes, not by viruses leaving and entering new cells. Cytoneme nanotubes are made of actin structures. Macrophages that are infected with viruses have a very large number of these tubes. Receptors near the junction of cells trigger the production of more of these nanotubes. Viruses create buds that travel along the nanotubes.
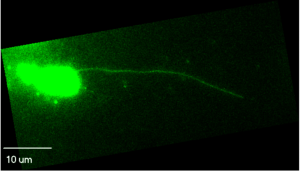 Actin nanotubes can be very long and produce widespread infections. Other viruses use larger filopodial extensions to send the virus even farther from the infected cell. These are stimulated by coordinated action of two virus proteins that commandeer the actin machinery.
Actin nanotubes can be very long and produce widespread infections. Other viruses use larger filopodial extensions to send the virus even farther from the infected cell. These are stimulated by coordinated action of two virus proteins that commandeer the actin machinery.
For very long distance spread somewhat larger tubes grow called comet tails that involve the function of both actin and microtubules. These large comet tails can send viruses rapidly for long distances. Without actin polymerization the spread of virus is limited. There are many different mechanisms for virus proteins to stimulate this travel in different sized extensions from the cell and in different ways.
A more dramatic way that the viruses travel is, also, related to actin structures. By interacting with p53 the cell death pathway can be triggered. These virus proteins interact with actin and the cell membranes. With radical changes in the actin blebs appear and then eventually the actin and myosin together damage the membrane causing cell death with an explosion of viruses into the environment.
Viruses don’t make actin or myosin. They produce proteins that interact with the many protein factors that trigger various acting functions and structures.
Virus Tricks Manipulate the Cytoskeleton
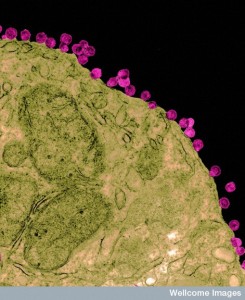 Many remarkable behaviors have been described for herpes, HIV and Ebola. With only 7 genes for Ebola and 9 for HIV and a small number of proteins, viruses somehow can compete and dominate large complex human cells, including the very immune cells that have many techniques to defeat them.
Many remarkable behaviors have been described for herpes, HIV and Ebola. With only 7 genes for Ebola and 9 for HIV and a small number of proteins, viruses somehow can compete and dominate large complex human cells, including the very immune cells that have many techniques to defeat them.
One of the remarkable mechanisms viruses use is manipulating and commandeering the actin cytoskeleton. Actin, with its myosin motors, is critical for all aspects of cellular function, particularly the shape, size, motility and migration of cells and organelles. Actin is particularly important in cellular signaling and neuroplasticity. These cytoskeleton fibers have been called the brains of the cell, since all function depends upon them and they are constantly building and rebuilding in response to mind.
But, where is the direction for the constant movement of the scaffolding molecules that respond instantly to mental events with dendrite spines and axon boutons? Another question is how can the virus, a tiny piece of genetic material know how to manipulate this actin behavior?
This is another example of the remarkable behavior of viruses with only a small number of genes and proteins. How can small pieces of DNA and RNA demonstrate such intelligent behavior? Where is the direction for this? Is this another example of mind interacting with molecules?